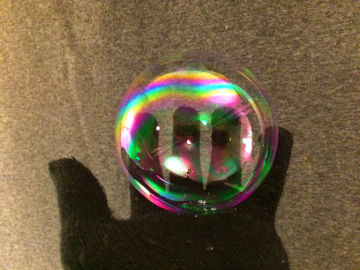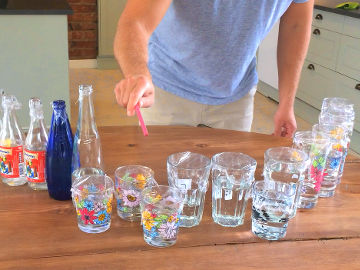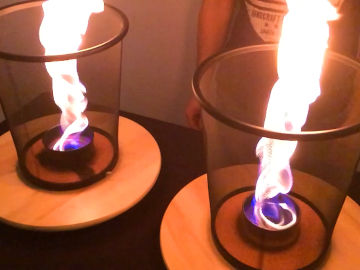Fun and easy science experiments for kids and adults.
Chemistry
Make a soap bubble float on invisible carbon dioxide. This is an experiment about chemical reactions, density, polar and nonpolar substances, and more.
| Gilla: | Dela: | |
Materials
- 1 large container (for example a large bowl, an aquarium or the sink)
- 1 cup
- Dawn (or Fairy) dish soap
- Glycerin (if you have some)
- Something to blow soap bubbles with (for example, the finger hole on a pair of scissors or something else that has a hole in it).
- Baking powder
- Water
Step 1


Step 2


Step 3


Step 4


Short explanation
When you mix baking powder with water, the invisible gas carbon dioxide is formed. Carbon dioxide is heavier than air and remains in the container. Soap bubbles are lighter than carbon dioxide and float on top of the gas.Long explanation
Something that plays a key role in the demonstration is chemical polarity. Chemical polarity is how electrons are spread, across a molecule or something else in chemistry. Molecules with an even distribution of electrons are called nonpolar. Molecules with an uneven distribution of electrons are called polar.Water consists of water molecules that attract each other quite strongly. The reason why water molecules attract each other is that they all have a positively charged end and a negatively charged end. Water molecules are polar. This can be compared to a bunch of magnets, which also have a positive and a negative end (these ends are magnetic instead of electrically charged, but the forces that arise work the same way). If these magnets were thrown in a bucket, they would line up, with positive ends against negative ends, and hold together. Other substances whose molecules are also polar mix easily with water. It's more difficult for substances that are nonpolar, i.e. whose molecules have no charged ends. Dish soap consists of molecules that have both a polar and a nonpolar end. These molecules can mix both with, for example, water (which is polar) and fat (which is nonpolar). When you blow a soap bubble, you push air (or another gas) into water. The water is pushed out to the sides, but still holds together thanks to its surface tension. The bubble that forms becomes spherical because this geometric shape has the least surface area and thus pulls the water molecules the least. A bubble of water would soon burst as the water evaporates into the air. However, by blowing bubbles of a mixture of water and dish soap, this can be remedied. In such a bubble, the layer of water is surrounded by a layer of dish soap on both sides. The dish soap molecules dip their polar end in the water and their nonpolar end in the air. The layers of dish soap delay the evaporation of water and the soap bubble lasts longer. Eventually, however, the bubble will burst, as water will still evaporate. The bubble also bursts if it hits something sharp, or grease that destroys the layer of dish soap. The secret to extra durable soap bubbles is to also add some glycerin. Glycerin attracts the water molecules, holds on to them and slows down evaporation. The mixture gets even better if it's allowed to stand for 24 hours, because the glycerin then has time to bind extra strongly to the water. When baking powder and water come into contact with each other, a chemical reaction takes place where carbon dioxide is formed. Baking powder consists of about 30 % baking soda, 40 % some acid (for example cream of tartar) and 30 % some moisture absorbing substance (for example corn starch). When baking powder and water are mixed, the baking powder begins to react with itself - bicarbonate and the baking powder's own acid reacts and forms a salt (which one depends on the acid) as well as carbon dioxide. Carbon dioxide in a gaseous state is heavier than air and therefore remains in the container - at least if you avoid creating drafts in the vicinity. The carbon dioxide is not visible because it does not absorb or reflect light, but it is there. You can detect where the carbon dioxide is by lowering a lit match into the container. It will be extinguished in the carbon dioxide, because there is no oxygen there. A soap bubble has a lower density than carbon dioxide and it will therefore "float" on top of it. This is due to the fact that the force of Earth's gravity has a greater effect on heavier things, in this case carbon dioxide, and pulls it closer to Earth's surface. If you have managed to create a durable soap bubble, which stays in the container for more than a minute, you will see that it both swells and sinks. This is because carbon dioxide diffuses into the soap bubble. A soap bubble is permeable for small molecules - both air and carbon dioxide can migrate in and out through its walls. Both air and carbon dioxide "strive" to be as common inside as outside the bubble. The phenomenon of substances spreading in this way only through their inherent kinetic energy is called diffusion. Air thus migrates out of the bubble to the environment outside where there is less air, and carbon dioxide migrates into the carbon dioxide-poor environment inside the bubble. However, carbon dioxide migrates in faster than air migrates out - because carbon dioxide, since it's a bit polar, gets through the bubble's largely polar wall more easily. Therefore, the bubble both swells and also becomes heavier (remember - carbon dioxide has a higher density than air).
Experiment
You can turn this demonstration into an experiment. This will make it a better science project. To do that, try answering one of the following questions. The answer to the question will be your hypothesis. Then test the hypothesis by doing the experiment.- How much carbon dioxide do you get from 1, 2, 3 etc. tablespoons of baking powder? Check the level in the container by lowering a lit match - where it goes out, the carbon dioxide starts!
- If you make a big soap bubble, does it float as well as a small one?
- What happens if you gently blow into the container when a soap bubble is lying there?
- What happens if you put a soap bubble in an empty container, and then pour the carbon dioxide over the bubble (yes, it is possible to pour carbon dioxide like water from one container to another)?
- Dip a stack of straws into the soap bubble solution and blow a cluster of bubbles. How does the cluster behave in the container of carbon dioxide?
Variation
Can you get smoke to settle as a layer on top of the carbon dioxide? This is a bit tricky, but results in a cool effect. Hold a burning piece of wood, immerse it in the container and let it go out in the carbon dioxide. Let the piece of wood smoke. Place something over the container while the piece of wood is smoking (but your arm must fit because you are holding the piece of wood), because otherwise the smoke is easily sucked out of the container. Some of the smoke is heavier than air but lighter than carbon dioxide so it will settle as a mysterious, but diffuse, mist in the container. The fog is best seen when hit by direct light.| Gilla: | Dela: | |
Similar
Latest
Content of website
© The Experiment Archive. Fun and easy science experiments for kids and adults. In biology, chemistry, physics, earth science, astronomy, technology, fire, air and water. To do in preschool, school, after school and at home. Also science fair projects and a teacher's guide.
To the top
© The Experiment Archive. Fun and easy science experiments for kids and adults. In biology, chemistry, physics, earth science, astronomy, technology, fire, air and water. To do in preschool, school, after school and at home. Also science fair projects and a teacher's guide.
To the top