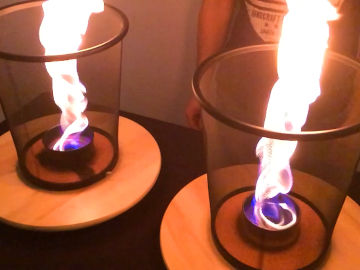Fun and easy science experiments for kids and adults.
Physics
Create heavy, cold, smoke. This is an experiment about why some things float and others sink.
| Gilla: | Dela: | |
Video

Materials
- 1 tall drinking glass
- 1 fork
- 1 lighter or matchbox
- 1 candle
- 1 pencil
- 1 newspaper page
- Tape
- Freezer
- Safety equipment: 1 fire extinguisher, 1 bowl of water, 1 pair of safety goggles
Warning!
These risks exist:- Something may catch fire.
- Someone may burn themselves.
- Inhalation of harmful smoke.
- Do the demonstration in the company of an adult with experience of fire.
- Wear safety goggles.
- Have a fire extinguisher ready.
- Have a bucket of water ready.
- Do the demonstration in a well-ventilated place.
- Never hold the paper tube with your fingers. Use a fork - and hold it as far out on the handle as possible.
- Do not touch the paper tube or inner part of the fork after the fire is out.
- Do not hold the flame too close to the cold glass. The glass may crack.
- Practice what to do if something catches fire or if someone burn themselves.
Step 1


Step 2


Step 3


Step 4


Step 5


Step 6


Step 7


Step 8


Step 9


Step 10


Short explanation
The smoke cools in the cold glass. Because of that it gets a higher density than air and sinks.Long explanation
Why does the smoke sink? When a substance is cold, it means that the particles (atoms, molecules or ions) the substance consists of only move a little and collide a little with each other. When a substance is hot, the particles move a lot and only collide with each other all the time, which means that they push away from each other and are on average far apart. In cold air, for example, the nitrogen and oxygen molecules are closer together than in hot air, and therefore cold air has a higher density than hot air. The temperature affects not only the density of gases, but also of liquids and - to a smaller extent - of solids. The density then determines the buoyancy of a substance. We all know that hot air rises (in other words, floats on cold air). But why really? Imagine all the particles that constitutes the air in the atmosphere (nitrogen molecules etc.). They move all the time, collide with each other and push on each other and their surroundings (temperature is a measure of this motion). If you put a certain amount of air in a balloon, you could measure how the air pushes on both the inside and outside of the balloon. This is the so-called air pressure. The air pressure can increase for two reasons; more particles gather in the same volume, or the particles get more kinetic energy (higher temperature). The particles of air have mass and thus they are pulled towards Earth's surface due to Earth's gravity. But because the particles collide with each other and thus constantly strive to spread as much as they can, they have, so to speak, an inherent resistance to gathering close together. Therefore, it is only close to Earth's surface that the air can become relatively dense, because this is where the gravitational force is strongest. Then the air becomes thinner the higher up in the atmosphere you get, as Earth's gravitational force becomes weaker. Therefore, the air pressure is highest at Earth's surface and becomes thinner the higher up in the atmosphere you get. There is another reason why the air is densest and the air pressure is highest at Earth's surface, also related to gravity. Because even if the gravitational force has not succeeded in pulling the particles higher up in the atmosphere all the way down to Earth's surface, it still causes these particles to push on the air below them. This pressure is equal to the weight of the air above. Now imagine a body in the atmosphere, such as a balloon with air in it (or a cloud of smoke as in this demonstration). The air pressure is thus always higher beneath the body than above. This means that the air beneath the body pushes the body upwards, more than the air above the body pushes it downwards. So every body that is placed in the atmosphere is automatically exposed to an upward force, called buoyancy or upthrust. How great this upthrust is, is described by Archimedes' principle: "a body in a liquid or gas is affected by an upward force, which is equal to the weight of the displaced liquid or gas". Most often, the body's own weight is greater than that of the displaced liquid or gas - that is, it has a higher density, and then it sinks. This is the case with the smoke in this demonstration. It has a higher density than the surrounding air and thus it sinks. This is a bit unusual, as we are used to smoke being hot and thus having a low density. For a body to float (rise) in the atmosphere, its density must be lower than the air in the atmosphere. An example is a hot air balloon. It also contains air, but this air has been heated. The air particles are further apart and the density is lower - it floats. However, the air pressure is as great inside as outside the balloon, which prevents it from being compressed. Although there are fewer particles inside the balloon, they compensate by colliding more violently with the inside of the balloon. Buoyancy works exactly the same in water (and all other liquids and gases) as in air. The only difference is that the particles there are water molecules. One can think of the atmosphere and the sea as a single coherent pillar of liquid/gas, with a sharp jump in buoyancy at the water surface. At the water surface, the surface tension (the fact that water molecules attract each other electrically) also plays a certain role, and can cause bodies with a higher density than water to actually float.Experiment
You can turn this demonstration into an experiment. This will make it a better science project. To do that, try answering one of the following questions. The answer to the question will be your hypothesis. Then test the hypothesis by doing the experiment.- What happens if you leave the smoke in the glass and just wait?
- What happens if you cover the glass with plastic wrap and just wait?
- What happens if you use a larger glass?
- What happens if you heat the glass instead (for example using boiling water)?
- Can you pour the smoke from one glass to another?
- Can you somehow get the smoke into water? What happens then?
| Gilla: | Dela: | |
Similar
Latest
Content of website
© The Experiment Archive. Fun and easy science experiments for kids and adults. In biology, chemistry, physics, earth science, astronomy, technology, fire, air and water. To do in preschool, school, after school and at home. Also science fair projects and a teacher's guide.
To the top
© The Experiment Archive. Fun and easy science experiments for kids and adults. In biology, chemistry, physics, earth science, astronomy, technology, fire, air and water. To do in preschool, school, after school and at home. Also science fair projects and a teacher's guide.
To the top