Fun and easy science experiments for kids and adults.
Chemistry
Put dry ice in a balloon and watch the balloon inflate. This is an experiment about states of matter.
| Gilla: | Dela: | |
Video

Materials
- Dry ice - About the amount of one sugar cube is needed for this experiment. Either block or pellets.
- If you buy a block:
- 1 towel
- 1 hammer (or something else to break the ice)
- 1 balloon
- 1 bowl
- Water
- Safety equipment: 1 glove or 1 pair of tongs
Warning!
These risks exist:- The freezing point of carbon dioxide is -78.5 °C (-109.3 °F), but carbon dioxide ice can be much colder than that. There is a risk of frostbite on contact with the skin. However, touching smoke or bubbles formed with the help of carbon dioxide is safe.
- Carbon dioxide sublimates (changes from solid form to gaseous form) in everyday temperatures, which results in a large amount of carbon dioxide gas that can push the air away. There is a risk of drowsiness, headache or, in the worst case scenario, unconsciousness or suffocation. However, if you only use one block of carbon dioxide and have normal ventilation, you don't need to worry.
- Never put dry ice in a closed container. There is a risk of explosion when the ice sublimates.
- Do not touch dry ice with bare hands.
- Make sure to have very good ventilation.
- Practice what to do if someone is injured by dry ice:
- Inhalation: Move to fresh air. Rest. Get medical attention if necessary.
- Skin contact: In case of frostbite, flush with water for at least 15 minutes. Use sterile bandage. Get medical attention.
- Eye contact: In case of frostbite, flush with water for at least 15 minutes. Use sterile bandage. Get medical attention.
- Ingestion: Get medical attention.
Step 1 (if you've bought a block)
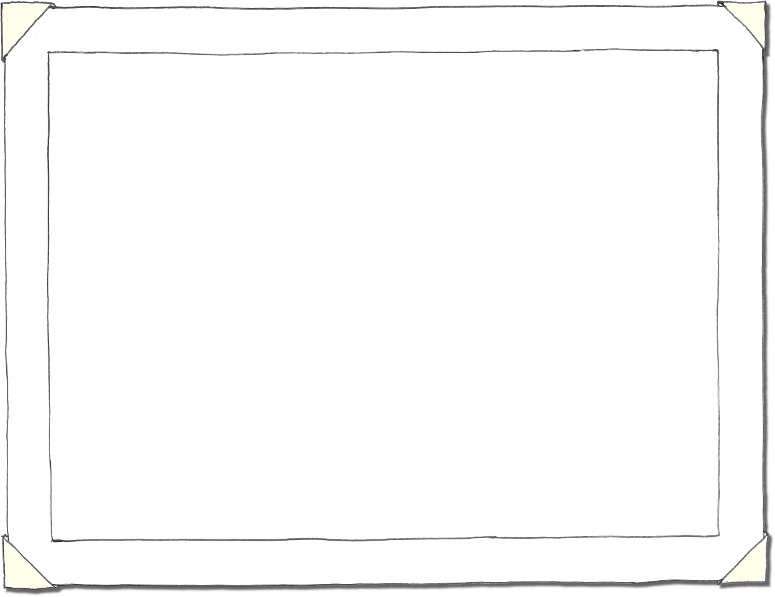

Step 2


Step 3

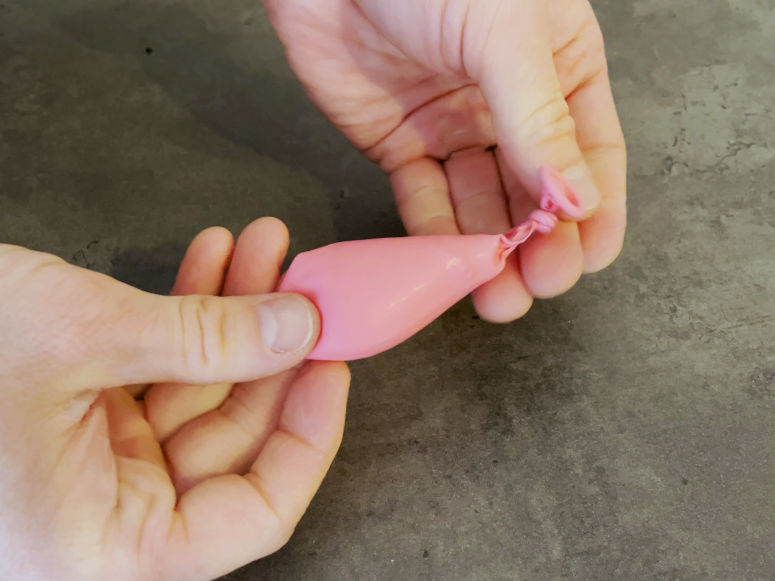
Step 4


Short explanation
Carbon dioxide sublimates at room temperature. That is, changes from solid to gas. This happens faster the warmer it is, and lots of carbon dioxide gas is formed. Here you capture the gas in a balloon that inflates. This demonstration also shows how the solid carbon dioxide just "disappears", without leaving any wet traces behind.Long explanation
Every pure substance can exist in different forms, each with distinct properties. These different forms are called states of matter. In everyday life, the three states of matter that are commonly observed are solid, liquid and gas. Then there are a few more that exist at extremely high or low temperatures, such as plasma, Bose-Einstein condensate, and quark-gluon plasma. In a solid state, the pure substance's particles (atoms, ions or molecules) are tightly packed and stuck together. In a liquid state, they are still close together, but can move relative to each other. In a gaseous state, they have completely separated from each other. The factors determining the state of a pure substance is pressure and temperature. At high pressure and/or low temperature, a pure substance is solid. At low pressure and/or high temperature, a pure substance is a gas. In between, the pure substance is a liquid. There are words for when a substance changes from one state of matter to another:- Melting: solid → liquid
- Sublimation: solid → gas
- Vaporization: liquid → gas
- Condensation: gas → liquid
- Deposition: gas → solid
- Freezing: liquid → solid
Experiment
You can turn this demonstration into an experiment. This will make it a better science project. To do that, try answering one of the following questions. The answer to the question will be your hypothesis. Then test the hypothesis by doing the experiment.- What if you use a smaller or larger bowl?
- What if you use colder or warmer water?
- What if you use more or less dry ice?
- What if you don't use a bowl of water, but instead just place the balloon on the table?
- What if you don't tie the balloon?
Variations
This demonstration can be done without hot water. It just takes longer.It's a bit tricky and risky to get an ice cube into a balloon (especially for younger children). A version of the demonstration is to put the ice cube in a bottle and thread a balloon over the opening.
| Gilla: | Dela: | |
Similar
Latest
Content of website
© The Experiment Archive. Fun and easy science experiments for kids and adults. In biology, chemistry, physics, earth science, astronomy, technology, fire, air and water. To do in preschool, school, after school and at home. Also science fair projects and a teacher's guide.
To the top
© The Experiment Archive. Fun and easy science experiments for kids and adults. In biology, chemistry, physics, earth science, astronomy, technology, fire, air and water. To do in preschool, school, after school and at home. Also science fair projects and a teacher's guide.
To the top