Fun and easy science experiments for kids and adults.
Chemistry
Let a metal wire cut straight through an ice block without the ice falling apart. This is an experiment about heat and states of matter.
| Gilla: | Dela: | |
Video

Materials
- 1 container of some kind (to freeze water in)
- 1 thin metal wire (for example a guitar string)
- Two 1.5 or 2 L plastic bottles
- Water
- Freezer
- Stuff for creating the setup (in this example 1 stool, 2 flower pots and 1 towel is used)
Step 1


Step 2

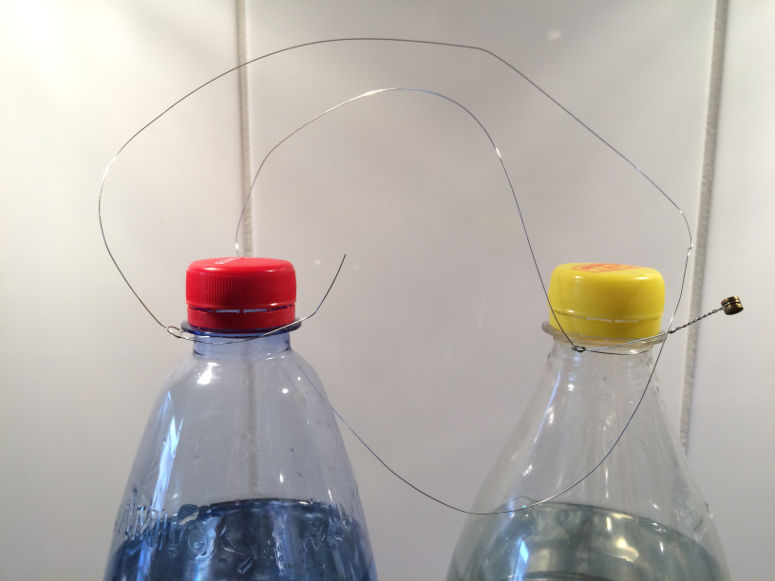
Step 3


Step 4


Short explanation
The wire conducts heat from the air into the ice. Therefore, it melts the ice around it. The water freezes again when the wire has passed.Long explanation
Temperature is a measure of how fast a substance's constituent particles move. In hot water, for example, the water molecules move around quickly, while in cold water they are slower. If the water molecules are slow enough, they attach to each other in a specific configuration - the water has frozen to ice. When two objects come in contact with each other, the temperature spreads between them. This is done by the particles in the warmer object colliding with the particles in the colder object and thus transferring kinetic energy to them. This transfer of kinetic energy through direct contact is called conduction. Energy that is being transferred through conduction - or radiation - is called heat. Some materials conduct heat better than others. This means that conduction is fast inside the material, but also to and from the material when it's in contact with another material. For example, metals are good conductors of heat, which has to do with how the atoms in metals are organized (more specifically, there are free electrons that can easily transport energy). In this demonstration, heat moves from the air, through the wire, to the ice around the wire. This causes the ice in proximity to the wire to melt and a layer of water to form. This water layer is thin - some researchers have suggested around 0.002 mm. As the wire is pulled downwards by the weights at each end, the water is forced up above the wire where it freezes on contact with the ice. At the same time, the wire continues to melt the ice beneath it. It's not certain that conduction is the reason why the wire moves through the ice, but it's the most probable hypothesis. Another hypothesis is that the pressure from the wire melts the ice - not the heat. Ice do melts due to increased pressure, something that is often used as an explanation for the formation of a thin layer of water under ice skates, making you glide so well. It is also used as an explanation for why you make great snowballs when you squeeze the snow. But when doing the math, this doesn't seem to add up, not for this demonstration, not for ice skating, and not for snowballs. The pressure just isn't high enough (however, is seems to be true when it comes to glaciers, to explain the layer of water that forms under them, on which they slide). Although conduction is the main explanation in this demonstration, pressure should theoretically still at least be a contributing factor, especially when the wire cuts through thin sections and all the weight from the bottles rests on a small surfaces of ice.Experiment
You can turn this demonstration into an experiment. This will make it a better science project. To do that, try answering one of the following questions. The answer to the question will be your hypothesis. Then test the hypothesis by doing the experiment.- What happens if you use heavier bottles, or pull down on the wire?
- What happens if you use a thicker wire?
- What happens if you use a fishing line?
- What happens if you do the demonstration outside when it's freezing?
- What happens if you do the demonstration under a warm lamp?
| Gilla: | Dela: | |
Similar
Latest
Content of website
© The Experiment Archive. Fun and easy science experiments for kids and adults. In biology, chemistry, physics, earth science, astronomy, technology, fire, air and water. To do in preschool, school, after school and at home. Also science fair projects and a teacher's guide.
To the top
© The Experiment Archive. Fun and easy science experiments for kids and adults. In biology, chemistry, physics, earth science, astronomy, technology, fire, air and water. To do in preschool, school, after school and at home. Also science fair projects and a teacher's guide.
To the top