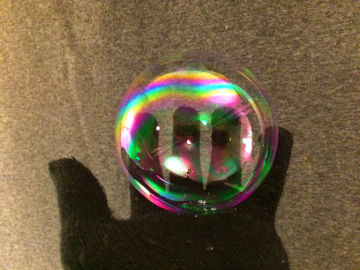Fun and easy science experiments for kids and adults.
Chemistry
Bounce and play with a soap bubble without it bursting. This experiment teaches you everything you need to know about polar and nonpolar substances.
| Gilla: | Dela: | |
Video

Materials
- Water
- Dawn (or Fairy) dish soap
- Glycerin (if you have some)
- 1 cup or drinking glass
- 1 pipette - or anything that has a hole you can blow through
- 1 pair of scissors
- 1 clean cotton mitten or glove
Step 1


Step 2


Step 3


Step 4


Short explanation
A soap bubble bursts when it touches fat or dirt. A clean mitten is relatively free from both.Long explanation
Water consists of water molecules that attract each other quite strongly. This means that water holds together. The reason why water molecules attract each other is that they all have a positively charged end and a negatively charged end. Water molecules are polar. This can be compared to a bunch of magnets, which also have a positive and a negative end (these ends are magnetic instead of electrically charged, but the forces that arise work the same way). If these magnets were thrown in a bucket, they would line up, with positive ends against negative ends, and hold together. Other chemical substances whose molecules are also polar mix easily with water. It's more difficult for chemical substances that are nonpolar, i.e. whose molecules have no charged ends, to mix with water. Dish soap consists of molecules that have both a polar and a nonpolar end. These molecules can mix both with, for example, water (which is polar) and fat (which is nonpolar). When you blow a soap bubble, you push air (or another gas) into water. The water is pushed out to the sides, but still holds together thanks to how the molecules hold on to each other. The bubble that forms becomes spherical because this geometric shape has the least surface area and thus pulls the water molecules the least. A bubble of water would soon burst as the water evaporates into the air. However, by blowing bubbles of a mixture of water and dish soap, this can be remedied. In such a soap bubble, the bubble of water is surrounded by a layer of dish soap on both sides. The dish soap molecules dip their polar end in the water and their nonpolar end in the air. The layers of dish soap prevent the evaporation of water and the soap bubble lasts longer. The worst enemies of a soap bubble are nonpolar substances (such as fat and oil) and dirt particles. Nonpolar substances pulls the protective dish soap molecules while the dirt particles, due to their sharpness, simply separate the water molecules. A surface that is free of nonpolar substances and dirt, for example a clean cotton mitten, is a good place for a soap bubble to rest on. Eventually, however, the bubble will burst anyway as water evaporates. How do you create the most durable soap bubbles? Here are some tips:- The secret to extra durable (tennis ball size) soap bubbles is to also mix in a little glycerin. Pour in half as much glycerin as dish soap. Glycerin attracts the water molecules, retains them and slows down evaporation.
- Let the soap bubble mixture rest for 24 hours before use. Then the chemical bonds have time to strengthen and the bubbles get stronger.
- Clean water is also important, as dirt prevents the water molecules from binding to each other.
- Also avoid blowing soap bubbles in the sun, as the heat makes water evaporate more quickly.
- The best soap bubble weather is after a rain, because the air is then full of water.
Experiment
You can turn this demonstration into an experiment. This will make it a better science project. To do that, try answering one of the following questions. The answer to the question will be your hypothesis. Then test the hypothesis by doing the experiment.- What other materials can be used to bounce soap bubbles on?
- Is it possible to color the soap bubbles with food coloring?
- What happens when two soap bubbles meet?
| Gilla: | Dela: | |
Similar
Latest
Content of website
© The Experiment Archive. Fun and easy science experiments for kids and adults. In biology, chemistry, physics, earth science, astronomy, technology, fire, air and water. To do in preschool, school, after school and at home. Also science fair projects and a teacher's guide.
To the top
© The Experiment Archive. Fun and easy science experiments for kids and adults. In biology, chemistry, physics, earth science, astronomy, technology, fire, air and water. To do in preschool, school, after school and at home. Also science fair projects and a teacher's guide.
To the top